Barbara Weber, Öko-Institut, Freiburg, Germany
A major problem in risk assessment of GE of plants and organisms in general is that the outcome of transformations cannot be fully foreseen. We have to deal with uncertainties in this field. Some of these can in principle be reduced by research.
If a transformant's phenotype differs obviously from the phenotype of the non-transformed parental plant, the transformant is probably not selected further for the breeding programme. But less obvious effects of the transformation are likely to be overlooked. For instance, slight differences in composition which may nevertheless have chronic nutritional or ecological effects may be overlooked. Generally, we can hope that risks which are caused by the engineered trait itself are more likely to be recognised. For instance, we are aware, or at least should be aware, of environmental risks if fitness enhancing traits are cloned (e.g. resistance to pests, diseases or abiotic conditions). We are, or should be, aware of health risks if the herbicide resistance mechanism protects the transgenic plant without degrading the herbicide. But even in such cases of possible risk awareness, predictions are seriously flawed because they deal with open systems and with open time scales. Therefore, real predictions on environmental and long term effects are unattainable, as noted in the earlier contributions. In addition, even the wanted and known effects of transgenes are not fully understood and cannot be controlled - we have already heard today about gene silencing.
Other uncertainties are caused by pleiotropy of genes. A gene is usually cloned because of an effect which the gene shows in the donor plant or organism, but it may have additional unwanted effects which were overlooked in the donor organism. The genetic background too of the recipient may influence effects of the cloned genes. More research in this field could help to detect some of the problems. Nevertheless, long term environmental and health effects cannot be completely foreseen. The side effects of transformations cannot be predicted at all. These are generated by undirected random integration of sometimes several copies of the transgene into the recipient's genome. Thus the question is: how should we deal with those uncertainties?
In a stringently precautionary approach one would avoid irreversible steps which might endanger future generations and one would dispense with releasing GMOs into the environment. But what has actually been done is that a political decision has been made not to dispense with releases and to accept inherently unpredictable risks. But it was claimed that by looking very carefully at GMO phenotypes all the risks which can be detected in this way would be avoided. As a consequence the principles of case-by-case assessment and proceeding step-by-step are acknowledged and supported by many researchers and these principles have been implemented in the surveillance procedures by regulatory authorities. At least this is what many researchers and regulatory authorities claim but, as I show later, this is not always the case.
In the following I should like to discuss how far thorough research into risks is really done and how far appropriate regulatory consequences are drawn. There are several estimations and studies which come to the result that ecological aspects were not even examined in nearly all field experiments which have been done until now. In most cases only agronomic traits were tested (Wrubel et al. 1992, Kareiva 1993, Mellon & Rissler 1995, Sukopp & Sukopp 1995). This means that the chance to learn something about the behaviour of transgenic plants in the environment was largely missed. Mellon and Rissler (1995) concluded that field experiments seem to be done following the motto 'don't look, don't find'. On the other hand, in those
cases where researchers really 'looked', meaning that they did appropriate experiments to reveal possible risks they tended to obtain evidence for risk scenarios. But then the results are often not taken seriously. A now well known example is the outcrossing behaviour of oilseed rape. Fully competitive progeny with turnips were produced more quickly and more effectively than some researchers in this field predicted (Scheffler & Dale 1994, Mikkelsen et al. 1996). Comparable results were found with squash (Curcubita pepo ssp. ovifera var. ovifera) and a free-living related species in the USA (Fuchs & Gonsalves 1997). Nevertheless, oilseed rape and squash have already been approved for commercial release, including lines with fitness-enhancing traits. Another example is the resistance of pests to plants containing Bacillus thuringiensis (Bt) toxin genes ('Bt plants'). Resistances in the case of some pests are now known to arise much faster than assumed hitherto (Gould et al. 1997). On the basis of recent findings cross resistances against different Bt endotoxins are expected to become a major problem (Tabashnik et al. 1997). Non-target effects of transgenic plants have not yet been investigated in most of the cases. But recent findings show that the population of soil microorganisms can be affected by, for instance, Bt cotton plants (Donegan et al. 1995). Bt corn seems to endanger beneficial insects (Schweizer et al. 1997). Proteinase inhibitors are cloned in, for instance, oilseed rape to render it pest resistant. Honey bees fed on sugar diets containing such inhibitors were shown to be adversely affected. However, it is not yet clear whether the concentration of the inhibitors needed to cause such effects are reached in the hive (Pham-Delègue 1997).
The possibility of horizontal transfer of recombinant genes from transgenic plants to microorganisms was always regarded as not yet proven experimentally, although horizontal transfer to fungi was published some years ago (Hoffmann et al. 1994). Recently horizontal transfer of recombinant genes from recombinant plants to bacteria has been shown experimentally. The researchers submitted a paper to Nature, but it was not accepted on the grounds that the findings were not surprising enough to be of interest (Smalla 1997).
Table 1: Viral recombination
between RNA-viruses before 1986 doubts if recombinations between RNA plant-viruses are possible at
all although it was known from animal viruses. |
between plant viruses and viral sequences in transgenic plants 1991 Lommel & Xiong and later several findings: |
assumption that selective pressure is essential although selective advantage of sequences acquired by supposed recombination during virus evolution is often not apparent |
|
| 1996 Wintermantel &
Schoelz: intraspecific recombinations without apparent or under moderate selective pressure |
|
doubts if recombinations may increase virus fitness although evolution of viruses supports this assumption |
|
| 1994 Fernandez-Cuartero et
al.: increased fitness through interspecific recombination 1997 Zhou et al.: interspecific recombination of DNA-Geminiviruses in the field increases fitness/virulence |
1996 Wintermantel &
Schoelz: recombinant viruses with increased fitness. (These are findings with the DNA-virus CaMV, but recombinations take place between RNA-molecules generated by reverse transcriptase.) |
| several findings: recombinations can be rather frequent under selective pressure: 3-36% recombinant viruses (Greene & Allison 1994; Wintermantel & Schoelz 1996; Maiss et al. 1997) without apparent or with moderate selective pressure: 13 % recombinant viruses (Wintermantel & Schoelz 1996) |
|
| assumption that sequence
homologies are important for frequent recombination but: interspecific recombination was not systematically searched for, may be supported by short, accidental sequence similarities. Non homologous recombination is regarded as the driving force of viral evolution by evidence gathered from sequence analysis of recent viruses. |
|
Another not so well known example which I would like to give here in more detail is viral
recombination (Weber et al. 1997, see Table 1 above). For a long time, recombination of
RNA plant viruses was thought not to happen. It was eventually demonstrated in 1986
(Bujarski & Kaesberg 1986). Recombination between infecting RNA viruses and virus
sequences in transgenic plants, i.e. with the messenger RNA expressed in those plants, was
shown in 1991 (Lommel & Xiong 1991). Then it was assumed that a strong selective
pressure is essential for RNA viruses to recombine, although this is not fully supported
by the analysis of the genomes of recent viruses. In 1996 Wintermantel and Schoelz showed
that recombinations between viruses and cloned virus sequences in plants can occur without
an apparent, or under a moderate, selective pressure. Thus, selective pressure is not an
absolute prerequisite for viral recombination in transgenic plants. Further, it was
believed that the recombinants would be unfit. But recombinants with increased fitness
were found in non transgenic (Fernandez-Cuartero et al. 1994) as well as in transgenic
plants (Wintermantel & Schoelz 1996). Zhou et al. (1997) found interspecific
recombination of a DNA virus which increased the fitness of the virus. It was a new very
harmful virus that came out of this recombination event. Recombinations can also be rather
frequent in transgenic plants (Greene & Allison 1994, Wintermantel & Schoelz 1996,
Maiss et al. 1997). Currently, it is often quoted that sequence homologies are necessary
for frequent recombinations. That means that only intraspecific and not interspecific
recombination would occur. But non-homologous recombination is regarded, from sequence
analysis of recent viruses, as the driving force for viral evolution (Simon & Bujarsky
1994). Probably the assumption that non-homologous recombinations are infrequent will turn
out to be false too. In my opinion, this story of short-lived assumptions about viral
recombination demonstrates above all how little is known about plant viruses and these
assumptions tend to underestimate the probability and risks of viral recombinations. In
this situation, a moratorium on releases of transgenic virus resistant plants would seem
to me to be a justified consequence.
Summarising so far, research into risk issues has been neglected in general and results supporting the existence and the probability of risks are not readily acknowledged by the scientific community nor the regulatory authorities.
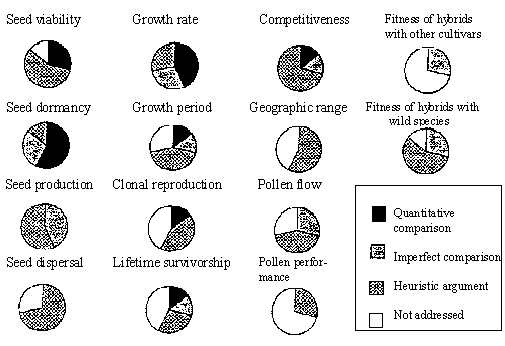
Up to now, I have concentrated on experimental field releases, and one might hope that risk assessment might be more thorough and conclusive when transgenic plants are tested for approval for commercialisation. But actually this is not evident from the first commercialisations in the USA. Companies wanting their transgenic crops removed from regulatory oversight by the USDA APHIS (United States Department of Agriculture's Animal and Plant Health Inspection Service) have to prove amongst other things that the transgenic crop is no more likely to generate a weed problem than its untransformed counterpart. Several characters contribute to weediness in a way not fully understood. Seed production, viability, dormancy, pollen flow and competitiveness are thought to be contributing factors. Purrington and Bergelson (1995) analyzed to what extent these properties had been examined by companies whose transgenic crops were approved for commercialisation (see Fig. 1 below). They classified the survey results according to a) valid quantitative experiments actually performed; b) poor experimental design, for instance no parental variety as control; c) heuristic arguments, e.g. weediness can be ascertained by examining the DNA sequence of the inserted fragment, and, not surprisingly, no weediness has been detected this way; finally d) weediness not discussed at all. In the second step Purrington and Bergelson (1995) examined whether the transformed lines, which were deregulated are really those which are eventually commercialised. Surprisingly, this is very often not the case. For instance, weak, highly inbred transformed lines were tested, but later on these individual lines are back-crossed and hybridised with other lines to produce varieties for the market. The latter were not tested in most of the cases.
Figure 1. Approved applications for nonregulated status for transgenic crops in the USA, categorised for their treatment of 14 issues relating to performance of plants containing transgenes. (from Purrington and Bergelson (1995))

So I come to the conclusion that, regarding ecological risks, case-by-case and step-by-step procedures are not really implemented. The researchers and regulators credo that the product and not the production process is decisive is not even adhered to by the researchers or regulators themselves. Furthermore, the real situation which will be coming up if transgenic plants are commercialised is not taken into account by research or regulation. I call this the multitransgenic plant scenario which is based mainly on two phenomena; 1) more and more transgenic plants are constructed, tested and approved which contain a growing number of different transgenes. Those may influence the plant's phenotype in a complex and interrelated manner but, to my knowledge, purposeful investigations into the interactions and synergisms between different transgenes in a plant have not been done. 2) Several transgenic crops with different engineered traits growing in the same region may exert combined effects on communities of organisms and ecosystems. The so-called 'superweed' is not out of reach if you imagine, for instance, a region where turnips are endemic and transgenic oilseed rape varieties are grown. Such varieties may comprise plants with different herbicide resistances and with resistances against pests, diseases, drought, salt, cold etc. Even if some of the characters mentioned here are not yet included in oilseed rape, we are currently approaching this 'scenario of multitransgenic plants'. In my opinion it should urgently be taken into account in research and regulation. Thus, what I conclude from what I have presented so far is that of course you may feel sure about the ecological safety of transgenic plants, but not on scientific grounds.
I move now to food safety because it is an especially controversial issue. On the one hand, transgenic plants and their food products are suspected to increase the problem of food allergies. On the other hand, GE is claimed to be capable of removing allergenicity from a known allergenic food. Transgenic plants often contain recombinant proteins which have not been food components until now. It is suspected that some of them would have an allergenic potential and that the probability of allergenicity of new proteins in food may be enhanced because these proteins did not take part in the process of selection and adaptation which has led to the current situation that some plants are consumed by human beings and are benign for them and others not. The probability of allergenicity is thought to be increased by some defence proteins of plant origin which are cloned to render plants more pest or disease resistant. Defence proteins like proteinase inhibitors or lectins are thought to be more likely to cause allergies than other plant proteins because of known adverse effects, or because of sequence similarities with known allergens (Franck-Oberaspach & Keller 1996). Finally, side effects of the transformation might alter the allergenic properties of the known allergenic food or might induce allergenicity in a hitherto non-allergenic food. These issues would be easily clarified if there were tests which could demonstrate the food allergenicity of a given food protein. However, this is only possible for known allergens which can be tested with the serum from people with antibodies to known allergens. How allergenicity of new allergens is to be tested is the real question.
What is actually done, or what may be done is shown with three comparisons (see Table 2 below): firstly, you may compare the sequence of a new protein to that of known allergens. This is in principle possible with linear but not with nonlinear epitopes. Epitopes are those sequences in an allergenic protein which are responsible for allergenicity. It is not possible to identify by sequence similarities nonlinear epitopes which are composed of different segments of the protein chain. In addition, really new proteins do not have to be similar to known allergens (Taylor 1994, Franck-Oberaspach & Keller 1996).
Table 2: Assessment of food allergenicity of proteins
1. Comparison with the sequence of known allergenic proteins
* is only possible with linear epitopes
* epitopes of known food allergens can be very different
* really new allergens are not supposed to be similar to known allergens
2. Comparison with the sequence of known not allergenic proteins
does not cover all possible epitope sequences
3. Comparison with other characteristics of known food allergens
molecular weight
of most recombinant proteins falls within the range of known allergens
glycosylation
is not a necessary prerequisite and not always decisive for allergenicity
resistance to heat treatment, processing and digestion
* fruit allergens are often labile to heat treatment and digestion
* Conalbumin, an allergen from eggs, is fragmented under simulated stomach conditions
* denaturation does not necessarily destroy allergenicity
* epitopes may be released by partial digestion
* the food matrix may protect allergens from proteolysis
* yoghurt and milk e.g. elevate the pH of stomach fluid in vivo for several hours
amount
* the major allergen from cod is a minor protein constituent
* allergic reactions may be elicited by traces of allergens e.g. by proteins in plant oils
* it seems that persons can even be sensitised by proteins in plant oils
Secondly, you can compare the sequence of the new protein to that of known non-allergenic proteins. But the non-homologous sequences of similar proteins may be large enough to contain several epitope sequences (Monsanto, 1994).
Thirdly, you may compare other characteristics of known food allergens with those of the new proteins, but the comparison of molecular weight does not allow the exclusion of allergenicity. Most recombinant proteins do not differ from known allergens with respect to molecular weight. Missing glycosylation does not preclude allergenicity. There are non-glycosylated food allergens. In addition, the sugar moiety of known glycosylated allergens is not always decisive for allergenicity (Komatsu et al. 1992, Lallès & Peltre 1996). Known food allergens are thought to be resistant to heat treatment, processing and digestion. But there are exceptions. Fruit allergens and an egg allergen, conalbumin, are readily digested but nevertheless allergenic (Lemke & Taylor 1994, Astwood et al. 1996). Denaturation does not necessarily destroy allergenicity. Further, epitopes may be released by partial digestion (Lehrer et al. 1996) and the food matrix may protect allergens from digestion (Taylor 1994). The stability of proteins may be enhanced by simultaneous digestion, for instance, of pH elevating yoghurt and milk (Martini et al. 1987). Food allergens are often assumed to be major components of allergenic food. But there again there are exceptions like the major allergen from cod which is a minor constituent of the proteins of this fish (Aas 1967, Aas & Elsayed 1969). Allergenic reactions may be elicited by traces of allergens, for instance by proteins in plant oils (Guéant et al. 1995). And it even seems that people can be sensitised by proteins in plant oils. Thus, all these tests and comparisons cannot prove that a given protein is a food allergen or not. If defence proteins are cloned, as I mentioned earlier, you must expect a higher allergenicity risk.
Finally, the tests to exclude harmful change of the allergenicity of known allergenic food which might be elicited by side effects of the transformation are flawed by the fact that allergenic food often contains several allergens. There are up to sixteen known allergens in soybeans and different individuals can be sensitive to different ones (Herian et al. 1990, Lallès & Peltre 1996). In the case of soybeans it was found that some allergic persons in the USA and Japan were allergic to different soybean proteins. For approval of Roundup Ready soybeans, Monsanto seems to have tested allergenicity with the pooled serum of five allergic persons originating from the USA (Burks & Fuchs 1995). Such a test is not fully reassuring even for all Americans who suffer from allergy to soybeans, let alone for allergic people in Japan and Europe.
Now I come to the question of whether GE can remove allergenicity from known allergenic food. Rice, like soybeans, contains several allergenic proteins. Different patients react to different rice allergens. In addition, the attempt to repress the expression of one of those allergens by the introduction of the antisense gene was not very successful. It was only reduced to 20-23% of the level in non-transgenic rice (Tada et al. 1996). As allergic persons may be responsive to only trace amounts of the allergen, and as rice contains several allergens, it has not yet been proven that GE can benefit allergic people and, on the grounds of the experiment cited, it does not even seem to be promising. I conclude that, as in the case of ecological risks, feeling sure about potential allergenicity of transgenic plants and food derived therefrom is not scientifically based.
Environmental and health safety have not been proven scientifically. Partly because of the lack of research and partly because the principles established by the scientific community and the regulatory authorities were not followed. Thus, what is needed is of course more and sometimes better research. And regulation should depend on research results as well as gaps in knowledge. This would lead to a more precautionary approach. I only want to mention that meanwhile simplified procedures for the approval of releases of transgenic plants have been implemented in the USA and Europe, a measure which seems not to be justified by results of scientific research. Partly environmental and health safety has not been fully proven because of gaps in knowledge which cannot be filled by more research. That is because we deal with open systems and open time scales and because predictions must be based on experiences which were obtained in the past. However, experience with GMOs is scarce and comparisons with non-transgenic organisms are flawed because comparability may not be given. A crucial problem is that the current state is not well known. How can you measure the effect of Bt plants if you do not know what the functions of the Bt genes are in the normal host, Bacillus thuringiensis? The function of these genes as well as the role in B. thuringiensis in natural communities is unknown. The same goes for antibiotic resistance and antibiotic producing genes in their hosts. Thus, the claims that the effects of transgenic organisms and food will be monitored to avoid adverse effects are not convincing. You cannot compare two things, one of which you ignore. The question is, how can we make the best possible decisions in a situation of uncertainty?
As scientists, we tend to extrapolate from our experience of how to design good experiments to making judgements. For example, as already mentioned, conclusive tests of transformed plants need to include non- transformed parental lines as controls. It is astonishing how often this apparently simple rule is not followed. If the transgenic lines behave like the parental ones with respect to weediness they are assumed not to cause greater problems than the non-transformed plant. In practice the same goes for the out-crossing behaviour of transgenics. Releases and even commercialisations were approved for transgenic plants which are assumed to outcross like their non-transgenic parents. These approvals were granted even for regions and countries where cross-fertile wild plants are endemic. But of course it is not the same if the transgenic crop or the non- transformed parent is spread or if recombinant genes or evolved plant genes are transferred. For instance, transgenes are usually regulated by constitutive promoters. Therefore foreign genes and their new hosts will not adapt and evolve further like natural genes and natural plants.
Scientist also tend to arrive at judgements from scientific experiments. But this is not justified at all. The definition of adverse and beneficial effects is not a law of nature (von Weizäcker 1997). As a consequence it cannot be a matter of science, at least not of life sciences, to give such definitions. Science and scientific information can only be a basis for judgements and decisions. Of course, scientific information should be the best possible and no false promises should be made. Likewise, it is not justified to give scientists the privilege of influencing political decisions. On the contrary, decisions on the implementation of technologies are political decisions which have to be made in as democratic a way as possible.
This holds all the more because the effects of a technology like GE of plants and food concern all citizens. Thus, some common criteria as to how to decide need to be found and discussed. One criterion of course is majority opinion. It is not so surprising that those engaged in GE tend to have different judgements about the technology compared with the majority of lay people. This is not or at least not only an effect of better knowledge. It was shown that the percentage of people rejecting GE food remains the same even when they are better informed. One study showed that better information had the effect of changing mainly the reasons for rejection (Trenker 1997). Supposedly it is a matter of different interests. For consumers, advantages of GE food and plants are less obvious than for researchers and companies working in this field. An especially strong argument in favour of gene technology that it could reduce unemployment has been recently disproved by one of the project managers of Prognos (Becher 1997). Prognos did several oft-cited studies on the effects of gene technology in Germany including effects on employment (Becher & Schuppenhauer 1996). Thus, rejection of GE plants and food by the majority of citizens is not irrational. On the contrary it is very reasonable. This judgement has of course to be respected. Persuasion to another judgement has to be brought about by arguments and not by insufficient labelling.
Another criterion for a decision on a given technology is reversibility. Human beings make errors and they ought to have the possibility of correcting them. Escaped transgenic organisms and recombinant genes are supposed not to represent reversible interventions in ecosystems and further evolution. But it is to be expected that they would be even less easily removable from the environment than chemicals or radioactive isotopes. They can not only be persistent and toxic as well as being disseminated in the environment like chemicals and radioactive isotopes, but - being living organisms - they can multiply, mutate and transfer their genes, and they can be or become pathogens.
We may propose 'error friendliness' as a further criterion. This term was coined by Christine von Weizsäcker who pointed out that a major problem of transgenic crops might be their success which might then accelerate the reduction of crop variability dramatically (von Weizsäcker 1976, von Weizsäcker & von Weizsäcker 1986).
GE also has to be judged with regard to its sustainability. For instance, Bt plants are resistant at best to one or a few insects for only a few years. The estimated period of time during which those insect resistances will work without being broken down by resistant insects tends to get shorter and shorter. This does not look like a sustainable technique. Further, transgenic plants will go on to need pesticides and inorganic fertilisers which are known to deplete natural resources, consume energy and pollute the environment. This is particularly obvious with herbicide resistant plants. But pest and disease resistant crops too, will need pesticides because they are not inherently healthy and resistant to all the pests and all the diseases which may attack them. Imagine fields of plants resistant to a pest which formerly attacked this plant. This is not only a challenge to this pest to break the plant's resistance. Additionally it is an optimal opportunity for hitherto minor pests to thrive on this crop and become a major pest. Sustaining the plant's health, as is the goal of organic agriculture, seems better for meeting the criterion of sustainability.
I plead for a discussion which aims at recognising and solving problems instead of being driven by a specific technique. If the public is asked to inform itself on GE and to discuss its opportunities and risks, it has indeed no choice. This is not very appealing. Of course everybody would prefer the opportunities rather than the risks. But for making sound decisions you always need a choice.
Thus the question might be posed: how can we provide enough healthy food and cause least harm to the environment? To answer this question you would consider all the possible ways to reach this goal. That way you would have the opportunity of making the best possible decision in the current situation.
References
Aas K. (1967) Studies of hypersensitivity to fish. Int. Arch. Allergy 31, 239-260
Aas K., Elsayed S.M. (1969) Characterization of a major allergen (cod) - Effect of enzymic hydrolysis on the allergenic activity. Journal of Allergy 44, 333-343
Astwood J.D., Leach J.N., Fuchs R.L. (1996) Stability of food allergens to digestion in vitro. Nature/Biotechnology 14, 1269-1273
Becher G. (1997) In: VDI-Nachrichten 21.2.1997
Becher G., Schuppenhauer M. (1996) Kommerzielle Biotechnologie - Umsatz und Arbeitsplätze 1996-2000. Einschätzungen der deutschen Wirtschaft. Arbeitsbericht der Prognos AG fùr das Bundesministerium fùr Bildung, Wissenschaft, Forschung und Technologie, Basel
Bujarski J.J., Kaesberg P. (1986) Genetic recombination between RNA components of a multipartite plant virus. Nature 321, 528-531
Burks A.W., Fuchs R.L. (1995) Assessment of the endogenous allergens in glyphosate-tolerant and commercial soybean varieties. Journal of Allergy and Clinical Immunology 96, 1008-1010
Donegan K.K., Palm C.J., Fieland V.J., Porteous L.A., Ganio L.M., Schaller D.L., Bucao L.Q., Seidler R.J. (1995) Changes in levels, species and DNA fingerprints of soil microorganisms associated with cotton expressing the Bacillus thuringiensis var. kurstaki endotoxin. Applied Soil Ecology 2, 111-124
Fernandez-Cuartero B., Burgyan J., Aranda M.A., Salanki K., Moriones E., Garcia-Arenal F. (1994) Increase in the relative fitness of a plant virus associated with its recombinant nature. Virology 203, 373-377
Franck-Oberaspach S.L., Keller B. (1996) Produktsicherheit von krankheits- und schädlingsresistenten Nutzpflanzen: Toxikologie, allergenes Potential, Sekundäreffekte und Markergene. In: Gentechnisch veränderte krankheits- und schädlingsresistente Nutzpflanzen. Eine Option fùr die Landwirtschaft? Schulte E., Käppeli O. (eds.), Materialien. Eine Publikation des Schwerpunktprogramms Biotechnologie des Schweizerischen Nationalfonds, Bern 1, 15-100
Fuchs M., Gonsalves D. (1997) Risk assessment of gene flow associated with virus resistant transgenic crop plants. In: Potential ecological impact of transgenic plants expressing viral sequences. Abstract, OECD workshop, 24-26 April, Gòdòllò, Hungary, 31
Guéant J.L., Moutété F., Olszewski A., Pons L., Gastin I., Moneret-Vautrin D.A. (1995) Allergie à l´arachide et à l´huile d´arachide. Rev. Fr. Allergol. 35, 312-319
Greene A.E., Allison R.F. (1994) Recombination between viral RNA and transgenic plant transcripts. Science 263, 1423-1425
Gould F., Anderson A., Jones A., Sumerford D., Heckel D.G., Lopez J., Micinski S., Leonard R., Laster M. (1997) Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens. Proceedings of the National Academy of Sciences of the USA 94, 3519-3523
Herian A.M., Taylor S.L., Bush R.K. (1990) Identification of soybean allergens by immunoblotting with sera from soy-allergic adults. International Archives of Allergy and Applied Immunology 92, 193-198
Hoffmann T., Golz C., Schieder O. (1994) Foreign DNA-sequences are received by a wild type strain of Aspergillus niger after co-culture with transgenic higher plants. Current Genetics 27, 70-76
Kareiva P. (1993) Transgenic plants on trial. Nature 363, 580-581
Komatsu S., Kajiwara H., Hirano H. (1992) Soybean seed 34-kDa oil-body-associated protein separated by two-dimensional gel electrophoresis. Plant Science 81, 21-27
Lallès J.P., Peltre G. (1996) Biochemical features of grain legume allergens in humans and animals. Nutrition Reviews 54, 101-107
Lehrer S.B., Horner W.E., Reese G. (1996) Why are some proteins allergenic? Implications for biotechnology. Critical Reviews in Food Science and Nutrition 36, 553-564
Lemke P.A., Taylor S.L. (1994) Allergic reactions and food intolerances. In: Nutritional Toxicology. Kotsonis F.N., Mackay M., Hjelle J.J. (eds.), Target Organ Toxicology Series, Raven Press, New York, 117-137
Lommel A., Xiong Z. (1991) Reconstitution of a functional red clover necrotic mosaic virus by recombinational rescue of the cell-to-cell movement gene expressed in a transgenic plant. Journal of Cellular Biochemistry (suppl.) 15A, 151
Maiss E., Varrelmann M., DiFonzo C., Raccah B. (1997) Risk assessment of transgenic plants expressing the coat protein gene of plum pox potyvirus (PPV). In: Potential ecological impact of transgenic plants expressing viral sequences. Abstract, OECD workshop, 24-26 April, Gòdòllò, Hungary, 23
Martini M.C., Bollweg G.L., Levitt M.D., Savaiano D.A. (1987) Lactose digestion by yogurt %- galactosidase: influence of pH and microbial cell integrity. American Journal of Clinical Nutrition 45, 432-436
Mellon M., Rissler J. (1995) Transgenic crops: USDA data on small-scale tests contribute little to commercial risk assessment. Bio/Technology 13, 96
Mikkelsen T.R., Andersen B., Jorgensen R.B. (1996) The risk of crop transgene spread. Nature 380, 31
Monsanto (1994) Application to the United Kingdom Advisory Committee on Novel Foods and Processes for review of the safety of glyphosate tolerant soybeans by the agricultural group of Monsanto Company prepared by Padgette R.S. et al., provided by Waters S.P.
Pham-Delègue M.-H. (1997) Risk assessment of transgenic oilseed rape on the honeybee. INRA, Laboratoire de neurobiologie comparée des invertèbres, 1-3
Purrington C.B., Bergelson J. (1995) Assessing weediness of transgenic crops: industry plays plant ecologist. Tree 10, 340-342
Scheffler J.A., Dale P.J. (1994) Opportunities for gene transfer from transgenic oilseed rape (Brassica napus) to related species. Transgenic Research 3, 263-278
Schweizer G. (1997) Genmais / Vergiftete Nùtzlinge. Facts 34, 94-95
Simon A.E., Bujarski J.J. (1994) RNA RNA-recombination and evolution in virus-infected plants. Annual Reviews of Phytopathology 32, 337-362
Smalla, K. (1997) pers. com.
Sukopp H., Sukopp U. (1995) Òkologische Modelle in der Begleitforschung zur Freisetzung transgener Kulturpflanzen. In: Òkologie transgener Nutzpflanzen. Albrecht S., Beusmann V. (eds.), Campus Verlag, Frankfurt/Main, New York, 41-64
Tabashnik B.E., Liu Y.-B., Finson N., Masson L., Heckel D.G. (1997) One gene in diamondback moth confers resistance to four Bacillus thuringiensis toxins. Proceedings of the National Academy of Sciences of the USA 94, 1640-1644
Tada Y., Nakase M., Adachi T., Nakamura R., Shimada H., Takahashi M., Fujimura T., Matsuda T. (1996) Reduction of 14-16 kDa allergenic proteins in transgenic rice plants by antisense gene. FEBS Letters 391, 341-345
Taylor S.L. (1994) Evaluation of the allergenicity of foods developed through biotechnology. In: Proceedings of the 3rd International Symposium on the biosafety results of field tests of genetically modified plants and microorganisms. Jones D.D. (ed.), University of California, Division of Agriculture and Natural Resources, Oakland, USA, 185-197
Trenker V. (1997) Kaum jemand würde "Gen-Food" kaufen. Badische Zeitung 22.09.1997
Weber B.E.G., Eckelkamp C., Jäger M. (1997) Ökologische Risiken gentechnisch veränderter virusresistenter Pflanzen . Submitted to publication in: Verhandlungen der Gesellschaft für Ökologie, Gesellschaft fùr Òkologie (ed.), 27
von Weizsäcker C. (1976) Vom Umgang mit der Gefahr. Manuskript und Vortrag bei der Evangelischen Studiengemeinschaft, Heidelberg 1976
von Weizsäcker C., von Weizsäcker E.U. (1986) Fehlerfreundlichkeit als evolutionäres Prinzip und ihre mögliche Einschränkung durch die Gentechnologie. In: Die ungeklärten Gefahrenpotentiale der Gentechnologie. Kollek R., Tappeser B., Altner G. (eds.), J. Schweitzer Verlag, Mùnchen, 153-161
von Weizsäcker C. (1997) nux 99, 8-9
Wintermantel W.M., Schoelz J.E. (1996) Isolation of recombinant viruses between cauliflower mosaic virus and a viral gene in transgenic plants under conditions of moderate selection pressure. Virology 223, 156-164
Wrubel R.P., Krimsky S., Wetzler R.E. (1992) Field testing transgenic plants. BioScience 42, 280-289
Zhou X., Liu Y., Calvert L., Munoz C., Otim-Nape G.W., Robinson D.J., Harrison B.D. (1997) Evidence that DNA-A of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination. Journal of General Virology 78, 2101 -2111