|
||||
|
|
||||
| Gold, the
sun-metal |
||||||
 |
Gold chloride only | |||||
| Kolisko’s gold-experiments require sunlight
to perform them. One rises a solution of gold (1% gold chloride) up a filterpaper in
diffuse daylight, preferably not direct sunlight. Gradually a photochemical (i.e.,
light-sensitive) reaction then takes place, so that colloidal gold precipitates. In 1927 in Stuttgart she performed a gold-experiment, during the solar eclipse of June 29th, with her gold solutions rising before, during and after the celestial event, and her first book reported on this result: 'On June 27th we have a normal picture of gold, whereas on June 28th it has become somewhat cloudy. Various specks and strokes have made their appearance and the picture looks almost dirty… The picture obtained at the time of the eclipse manifests the above-mentioned phenomenon still more strongly. A large number of specks have appeared. The colours are not so luminous as on other occasions; their tones are mostly a brownish-red, dirty violet. It is altogether an unpleasing picture …On June 29th at 5.19 p.m. [after the eclipse] the picture of gold has become quite clear again; the colours are more luminous but they have not yet assumed their natural, inherent beauty and purity.' (1). It took just over a day for the gold to resume its normal hue. She also mixed solutions of tin and gold, as produced the ‘so-called gold-purple of Cassius …. There are times when the most wonderful gold-purple appears, immediately solutions of tin and gold are poured together and again there are times when absolutely nothing is to be seen’ [nb I, N.K., could never get this]. Her general comment was, ‘The pictures of tin and gold have, as a rule, something extraordinarily pure and delicate about them’
Mixing tin and gold chloride solutions (by N.K.) On June 27th a ‘light purple’ hue was appearing and on the 28th it was violet. Then during the eclipse on the 29th , ‘Instead of a beautiful picture with tones of yellow and violet produced by the gold in which are inscribed the workings of the tin-forces, there is merely an upward suction of empty water, as it were. The gold has been dragged down to the bottom and appears as a thick, black line.’ (2) It took some days for the colours to reappear. Many eclipse experiments were done but never published: ‘Every such occurrence, even though it may be hidden by clouds, or invisible from the place where the experiment is being made, infallibly inscribes its effect in the metal salt patterns on the filterpaper. All the eclipses of the sun and Moon in 1927 and 1936 were observed and the experiments showed the characteristic reaction in every case.’ These experiments re-established the ancient notion of correspondences: ‘We are thus able definitely to state that these striking and characteristic changes in the filter patterns only occur at the times of the celestial phenomenon that furnishes the reason for the experiment, and only with the metal that corresponds to the planet or planets affected.’(3) Kolisko went to Bursa in Turkey in 1936 (with Elizabeth Vreede) taking apparatus to checkout the path of a total solar eclipse. Her gold chloride Steigbild of June 18th (the day before the eclipse) showed that ‘the bright, animated colouring of the middle portion is pale rose-violet.’ Just before the eclipse, ‘In the lower past of the pattern, which on the previous day showed clear yellow, appeared four dark violet spots with small radiating lines.’ During the eclipse on the 19th, ‘The gold solution, which normally expresses its nature in cheerful colours becomes dull and drab, we could clearly see the powers of darkness working themselves into the gold. When we work with gold we could say that we have a bit of the Sun in our hands, Sun forces which have condensed into earthly substance’ (4) The three pictures below are from her book, Gold and the Sun. During the eclipse, she reckoned, ‘The darkening which the Sun experiences in the cosmos is also experienced by the gold fluid on the earth and this is revealed in these experiments.’ A day later in the aftermath a peach-blossom colour appeared (next figure). Kolisko burnt all her archive material in 1975 so nothing remains beyond what she published. It looks to me as if some other solution has first been risen to make this image, then the gold afterwards.
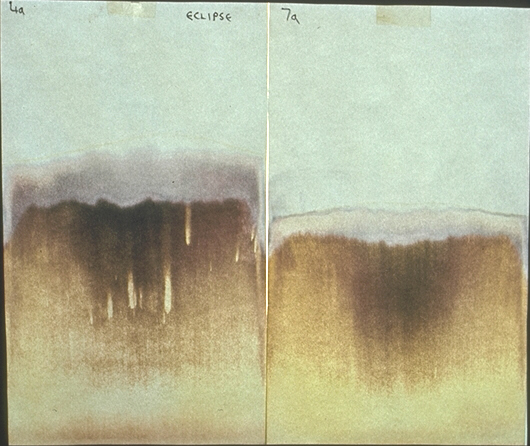
(These images, copyright Andrew Clunies-Ross, grandson of L.Kolisko, are reproduced with kind permission). I (N.K.) preferred to do a gold experiment when the eclipse was not visible, as I was not aiming to obtain results due to any darkening of the sky. The picture below shows two gold images I made, one during an eclipse, sometime in the 1970s (I omitted to record the date), as may remind one of the above comments by Kolisko.
Gold chloride during an eclipse (N.K.) Here’s a fine quote from Wilhelm Pelikan on gold: 'Gold has a tremendous "intensity of being life". This is shown by phenomena of light and colour. The serene splendour, the shining magnificence of the impression that the sight of gold makes upon our minds and senses is the highest enhancement of which the brightest of all colours, yellow, is capable. There is an 'inner heaviness' in the yellow of gold; it gives itself weight. A dilution on 1:100 million still makes water visibly purple. The beautiful red glass in cathedral windows is due to the addition of a trace of gold when the glass was poured' (5) Silver’s response to a lunar eclipse An experiment with colloidal silver was performed in 1997 by Michael Theroux, then editor of the Californian journal, ‘Borderlands.’ Theroux performed a quantitative checkup on Kolisko’s purely qualitative approach. He prepared solutions of ‘electrochemically produced colloidal silver’ before, during and after a lunar eclipse. Colloidal silver is used for medical purposes and he obtained two sets of apparatus for preparing it. He used these over the lunar eclipse of 23rd March, 1997 he used two sets of apparatus. He sent his samples off to a laboratory for the measurement. From its standard level of 6000 to 8000 parts per billion, ‘the amount of silver began to decrease nearing the eclipse, with a reduction to 1900 ppb during the eclipse.’ He concluded, ‘It is obvious that these associations indicate that the entire process of the electrochemical production of colloidal silver is ruled by lunar influence’ (6). One hopes that some repeat of this experiment is attempted. References L.Kolisko, ‘Working of the stars in earthly substances. The Solar Eclipse June 29th
1927,’ Stuttgart 1928, p.7 |
||||||

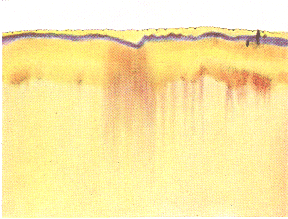 18 June 1936, 05.52
18 June 1936, 05.52 19 June 1936, 05.52, eclipse
19 June 1936, 05.52, eclipse 20 June 1936, 05.52
20 June 1936, 05.52