|
|
||||
| Experimental Procedure | ||
| The Sun produces gold; the Moon
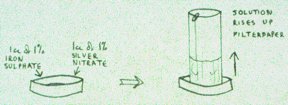
silver; Saturn lead; and Mars iron. Proklos of Byzantium, 5th century A.D. Some more practical details may not come amiss, for readers who wish to get their hands on some equipment. The paper: cut out a couple of dozen rectangles of the size required - 15 cm wide will fit snugly into the dishes - holding them at one end, their 'top' end - then roll them all together into a cylindrical shape. Take them out as required and clip them with paper clips, standing them upside down until the solutions are ready. A general purpose grade filterpaper ('Whatman's number one') is suitable. The dishes: petri dishes can be used, or get special 'steigbild' dishes (manufactured by the Lukas Klinic, Arlesheim, Switzerland), designed so that all the solution rises up the filterpaper. Also, cylinders of filterpaper stand up better in them. The chemicals: obtaining the chemicals for a private citizen is far from easy, and your best bet may be to find a school chemistry teacher who will let you have some. The pure 'Analar' grade ferrous sulphate is necessary, which is a pale green colour and not brown. While you're at it, if you can borrow a digital Ph-meter (which is not expensive) this will conveniently show any hydrolysis of the ferrous sulphate during the course of the experiment. Mixing: ordinary pipettes can be used to suck up the required amounts of the solutions which are then mixed in the dish. However, an adjustable pipette which will suck up and deliver the required amount is easier; or, better still, automatic pipettes fitted to a flask, so that as the top is depressed the required amount is delived. Such automation standardises the mixing procedure. If a lead solution is required, then the silver nitrate and lead nitrate can be placed together in a flask adjusted to deliver 3 ml (or whatever) each time. Timing: as the solutions are allowed to rise up the filterpapers in a dim light, I suggest noting when the first black speck of silver appears on your filterpaper, which becomes the nucleus or seed from which a 'form' grows. This will normally be a few minutes for the iron/silver mixture, but will be quite a bit longer if lead is also present. If a black precipitate of silver forms at the base of the paper around the dish within a minute or so, discard as it means that the dish is dirty. The pictures: Try the procedure a day or two before you wish to begin, to see if the forms are turning up properly. Sometimes they just don't come, which is a problem. In this situation one can vary the concentrations of the chemicals used, for example more iron will make the silver precipitate more quickly. If the forms still don't appear, give up. There's no point going through an experiment if the proper (slightly three-dimensional-looking) forms are not manifesting at the start. The phenomenon is a bit like the clouds in the sky: they change in shape from day to day, and I don't know why. Perhaps after all, as Kolisko said, this reflects changing conditions in some etheric-force ambience. Photography: photographing the papers is optimal using reflected and transmitted light. So, put the pictures onto glass inside the house, with a black paper surround, and photograph while illuminated both from behind and in front. Dry filterpapers using 'silica gel' crystals. NK
|
||